Research Goal:
Whether from a viral infection or some sort of cellular dysfunction, cytosolic dsRNA is an indication that something is not right in the cell. Research in the Ramsey Lab centers on a family of innate immune receptors called the RIG-I-like receptors (RLRs) which are responsible for sensing the presence of cytosolic dsRNA and initiating an innate immune response. Our approach to studying the RLRs is inspired by comparative immunology. We believe that by biophysically characterizing RLRs from many different species we will discover fundamental aspects of their functions as well as identify plasticity in their mechanisms which have allowed them to evolve and occupy different ecological niches. By combining our molecular biophysical investigations with cell-based functional assays, our work will provide a holistic perspective on RLR function and inform on the design of therapeutics from novel antivirals to vaccine adjuvants and cancer immunotherapies.
Why should we study RLRs?
RNA viruses comprise some of the most significant health threats across the world for both humans and animals. The innate immune system constitutes the body's first line of defense to invasions by these pathogens. The RIG-I-like receptors (RLRs) are a family of pattern recognition receptors which are responsible for sensing cytosolic double-stranded RNA (dsRNA), a highlight of viral infection, and initiating an innate immune response. Proper function of RLRs is required for a robust initial defense against viral infection. Furthermore, RLRs are a common target for vaccine adjuvant design and are even emerging as potential targets for therapeutics which sensitize certain tumors to novel immune checkpoint inhibitors. It is critical that we understand the precise biophysical mechanisms underlying RLR function in order to inform the design of therapeutics which target RLRs.
What do we know about how RLRs function?
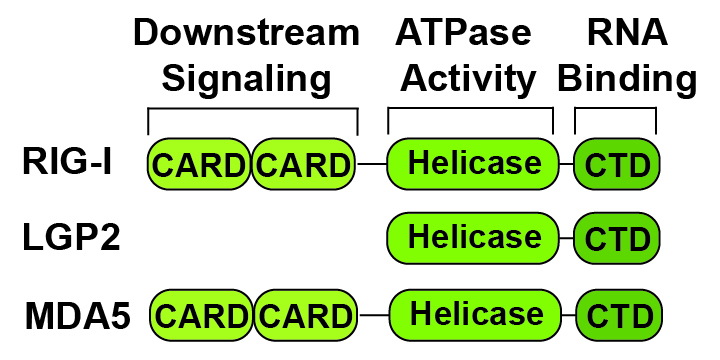
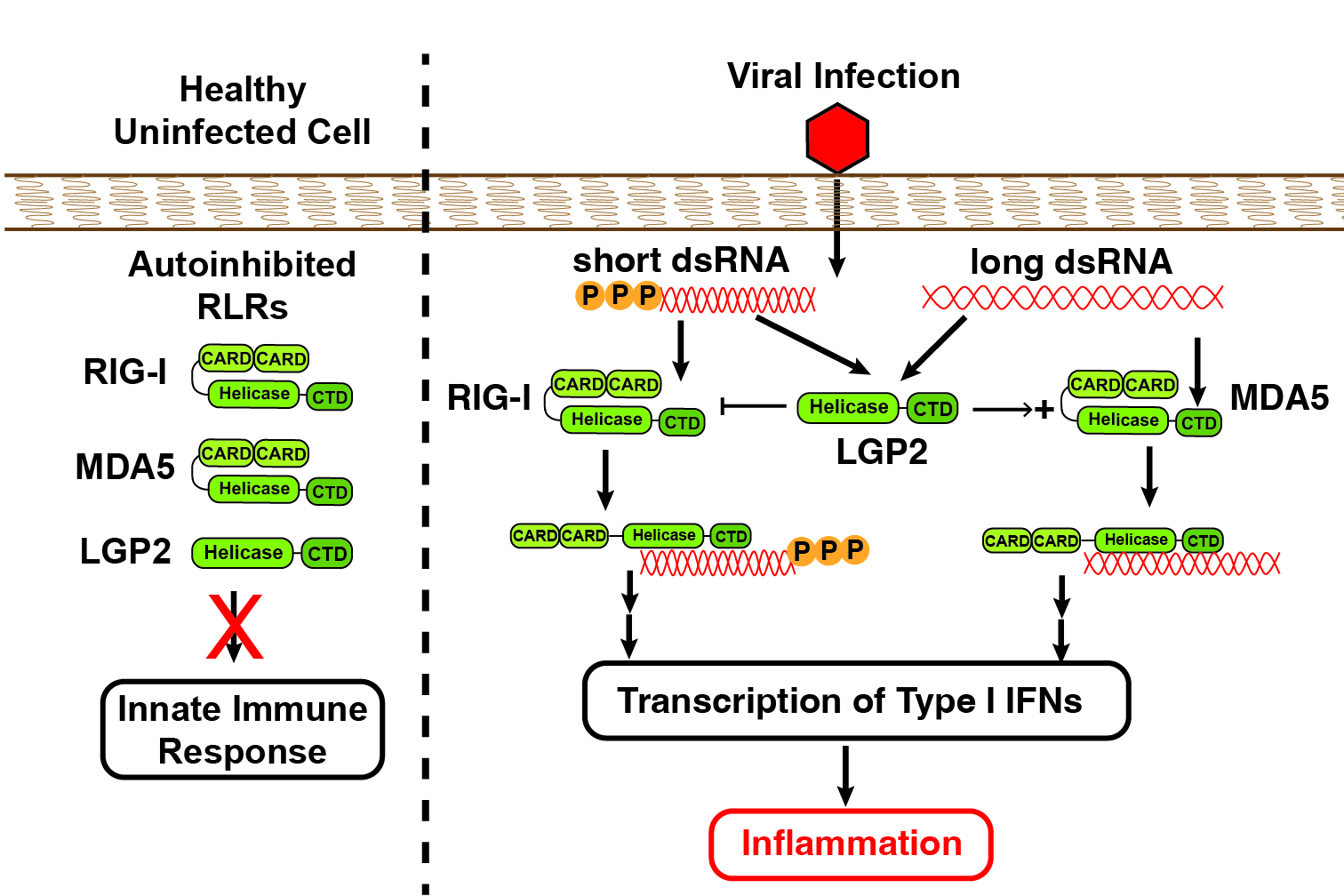
RLRs are multi-domain proteins regulated by inter-domain interactions and large-scale conformational changes. In the absence of cytosolic dsRNA, RIG-I and MDA5 exist in autoinhibited states wherein intramolecular interactions between the helicase and caspase activation and recruitment domains (CARD) block subsequent interactions. This autoinhibition is released upon dsRNA binding to the C-terminal and helicase domains allowing downstream signaling and the induction of inflammation.
RLR Signaling
The RIG-I-Like Receptors